Eindhoven
University of Technology
Department of
Applied Physics
Group
Elementary Processes in Gas Discharge
N-Laag, G2.04,
5600MB Eindhoven
e-mail:
mfgendre@tue.nl
web site:
http://www.geocities.com/mfgendre
Light,
and ways of producing it, undoubtedly belongs to the most fascinating and
exciting kind of science man has ever tried to master. To be more exact,
light sources do not belong to one kind of science but embody most of them.
This is the development of vacuum techniques, of particular glasses, the
purification of gases, the refinement of metals, the elaboration of
fluorescent substances, and other countless engineering feats that allowed
the making and improvement of all lamps we depend on today.
Of
course, many of these breakthroughs were precisely driven by the need for
better light sources, having longer lifetimes, higher efficiency and
better color properties. Yet, no one suspects that two centuries of
scientific research, discoveries, developments and refinements stare upon
us every time we flip a switch to give birth to light.
It was
exactly two hundred and one year ago that Humphry Davy set the foundations
of the lighting industry with his simultaneous discoveries of light
emission from incandescent metal wires and from electrical arcs (also by
W. Petrov). Until 1802, and since 400,000 BC, man had relied solely on
fire for his lighting needs.
The
invention of the electric pile by Alessandro Volta in 1800 opened a brand
new era of perspectives. His stacks of copper, zinc and saltwater-soaked
cardboards allowed the circulation of steady flows of electric currents
that would eventually spark the lighting revolution. A revolution that was
indeed slow to start.
The
early years
The
discoveries of Davy and Petrov had to wait five decades, the development
of steam-powered dynamos and the refinement of Volta’s battery, before
becoming a practical reality. By 1850, Léon Foucault built the first
carbon arc lamp that was subsequently used for theatrical lighting, while
four years later Einrich Goebel, a German emigrant in the USA, made the
first practical incandescent lamps. His sources were made of carbonized
bamboo filaments enclosed in evacuated perfume bottles, and were intended
to illuminate the shop window of his watch shop in New York city.
A third
way of electric lighting emerged in 1856 from the discovery by Michael
Faraday (England) of the electric glow discharge in rarefied gasses
(1831-35). This year, Julius Plücker and glass blower Enrich Geissler
started some systematic investigations of electrical discharges in
evacuated glass tubes provided with electrodes at each end. Subsequent
experiments from Hittorf, Crookes and Golstein revealed that the light
color of the discharge changed upon the addition of other gases and vapors.
This phenomenon was finally understood in 1859, when Robert Bunsen and
Gustav Kirchoff showed that each chemical element emits a specific set of
light colors, or spectral lines. This discovery eventually set the
foundations of spectroscopy. However, the inner working principles of
these tubes were not understood until the 1920s, when General Electric
(GE, USA) scientist Irving Langmuir studied and made the first accounts of
the physics of ionized gases, and coined the term plasma to
describe them. Then for this reason and others, “Geissler” and “Crookes”
tubes were relegated to the rank of lab curiosities until the beginning of
the twentieth century.

On the
side of carbon arcs, many improvements followed the lamp of Foucault. From
the work of Foucault and Dubosc, Serrin designed in 1859 a mechanical
system to keep the arc at a given position despite the unequal burning
rate of the cathode and the anode. Later, Crompton in England and
Wallace-Farmer in the USA made an arc lamp that was regulated in voltage,
thus permitting its use in series circuits. A further major step followed
in 1870, when Russian engineer Paul Jablochoff invented a self-regulating
arc lamp made of two close graphite rods separated by a layer of plaster
of Paris. These lamps had a lifetime of 90 minutes, and a set of
electrodes could not be re-ignited once it has been used. Despite its many
drawbacks, this kind of source led in 1878 to the first practical electric
arc street lighting in Paris. Two years later, Compton and Pochin in
England and Friedrich von Hefner-Alteneck in Germany invented the
differential carbon arc lamp, which was power-regulated by monitoring both
arc current and voltage. This system eventually superseded Jablochoff’s
lamp in street and industrial lighting.
Carbon
arc systems were pretty crude, cumbersome, noisy, dirty and drew a lot of
electrical power.
Beside
this, its bright harsh light did not make it suitable for home lighting.
The consequence is that many persons looked for a better and softer way of
producing light, and it was already of common knowledge that a piece of
carbon or metal heated by a current would do the job. However, things
sound far simpler than they are, and most of the attempts went up in smoke
as all materials eventually caught fire. The culprit was not so much the
filament material than the poor quality of vacuum in early lamp
prototypes.
Emergence
and development of practical incandescent lamps
The
development of incandescent filament lamps owes a lot to that of vacuum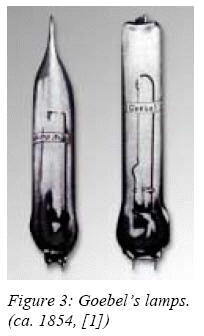
pumps.
In 1838 it was discovered that carbon brought to incandescent does not
consume in a air-free environment. From this knowledge the enclosed arc
lamp was born in 1893 (Jandus and Mark) and had a lifetime of 150 hours,
or three to five times that of lamps burning in free air. Although it was
known that platinum wires could be brought to incandescence in open air
for a long time (de la Rue, 1802), the need for lamps with higher filament
temperatures was felt. Carbon rods were studied and used by J.W. Starr and
M.J. Roberts between 1840 and 1854. The former made in 1845 a lamp
partially evacuated with a mercury column from Torricelli’s barometer.
Lodyguine, a Russian scientist, circumvented in 1856 the problem of poor
vacuum by using an atmosphere of nitrogen instead. Two hundred of his
carbon rod lamps were successfully used for lighting the harbor of St
Petersburg. These first lamps, although successful in their own right, did
not show a good lifetime due to the presence of residual impurity gases
either in nitrogen or in vacuum. Two major breakthroughs speeded up the
development toward a commercially viable lamp. First, in 1865 Sprengel
invented the mercury-drop vacuum pump, which was much better than von
Guericke’s pump developed around two hundred years before. This new device
could evacuate a vessel down to at least a ten thousandth of the
atmospheric pressure (10 Pa), a factor hundred lower that previously
achieved. L. Boem then improved this pump in 1878, and reached a millionth
of atmospheric pressure (10 mPa).
However, no matter how good lamps were pumped down, their lifetime was
still too short (several hours at best). The reason for this was
discovered in 1879 by Francis Jehl and Thomas Edison (USA), who found that
gases occluded in lamp materials are released in vacuum over time. They
then patented an effective outgassing method, which consisted of heating
the lamp during the pump-down process. In February of this same year,
Joseph Swan demonstrated a working incandescent graphite rod lamp before
the Royal Institution in Newcastle, England. This was eight months before
Edison made his successful low resistance carbon filament lamp.
Historically, Swan was the first to achieve a working carbon incandescent
lamp. However, the lamp lifetime was reportedly too short to be
commercially viable, which was not the case of Edison’s lamp. Edison
primarily used a U-shaped carbonized cotton thread for the filament, later
replaced by a carbonized bamboo fiber which boasted a luminous efficacy of
2 lm/W (ten times lower that today’s standard filament lamps) and a
lifetime of 45 hours.
By the
end of the 1870’s, the principles for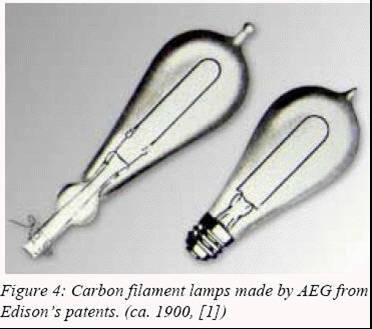 making
a good incandescent lamp were established, and it was then agreed that a
low resistance filament was needed for its use in parallel circuits. This
set the requirements for thinner filament, which are prone to burn out
quickly in poor vacuum. A better lamp thus called for stringent
improvements of the making procedures and the quality of the materials.
Then from 1880 to 1883 many inventors worked at improving the quality of
the carbon filament. Swan came up with a novel process that consisted of
squirting reconstituted cotton into threads, which were carbonized into
very fine carbon filaments of constant diameters. In 1894, A. Malignani
introduced the use of red phosphorus as a chemical getter, which maintains
an excellent level of vacuum in the bulb throughout the lamp life.
making
a good incandescent lamp were established, and it was then agreed that a
low resistance filament was needed for its use in parallel circuits. This
set the requirements for thinner filament, which are prone to burn out
quickly in poor vacuum. A better lamp thus called for stringent
improvements of the making procedures and the quality of the materials.
Then from 1880 to 1883 many inventors worked at improving the quality of
the carbon filament. Swan came up with a novel process that consisted of
squirting reconstituted cotton into threads, which were carbonized into
very fine carbon filaments of constant diameters. In 1894, A. Malignani
introduced the use of red phosphorus as a chemical getter, which maintains
an excellent level of vacuum in the bulb throughout the lamp life.
The
search for higher luminous efficacies and color temperatures pushed the
research toward higher filament temperature. Besides a shortening of their
lifetime, this led to the severe blackening of lamp bulbs as carbon has a
high vapor pressure. Then, more refractory filament materials were needed
in order to reach more than 1200ºC. In 1893, Lodyguine investigated
several metals, which included tungsten, while four years later Carl Auer
von Welsbach succeeded at making an osmium filament lamp that was put on
market in 1902. Followed in 1905, Dr Hans Kuzel made the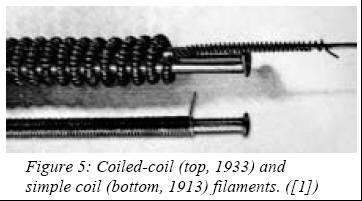 first
(brittle) tungsten filaments, which were used in new lamps marketed the
year after. This novel source pushed the lifetime up to 1000 hours and had
an efficacy of 8 lm/W (two times that of carbon filament lamps), which
eventually put an end to the osmium lamp of Auer von Welsbach. In 1907,
these lamps were also made to operate on 110V mains and were available up
to the 500W size. The next major breakthroughs happened from the work of
William Coolidge (General Electric, USA), who in 1910 succeeded at making
ductile tungsten filaments (as opposed to those made until then). Because
of its higher mechanical strength, this filament could be operated at a
higher temperature, thus boosting the lamp efficacy to 10 lm/W. Two years
later, Langmuir discovered the benefits of coiled tungsten filaments
operating in inert atmospheres (nitrogen, then argon-nitrogen mixture).
The winding permitted a reduction of the filament thermal losses, while
the surrounding gas lowered its evaporation rate. Both combined, this gave
a lamp efficacy of 12 lm/W (first marketed by GE in 1913 in 500, 700 and
1000W sizes) and spelled
first
(brittle) tungsten filaments, which were used in new lamps marketed the
year after. This novel source pushed the lifetime up to 1000 hours and had
an efficacy of 8 lm/W (two times that of carbon filament lamps), which
eventually put an end to the osmium lamp of Auer von Welsbach. In 1907,
these lamps were also made to operate on 110V mains and were available up
to the 500W size. The next major breakthroughs happened from the work of
William Coolidge (General Electric, USA), who in 1910 succeeded at making
ductile tungsten filaments (as opposed to those made until then). Because
of its higher mechanical strength, this filament could be operated at a
higher temperature, thus boosting the lamp efficacy to 10 lm/W. Two years
later, Langmuir discovered the benefits of coiled tungsten filaments
operating in inert atmospheres (nitrogen, then argon-nitrogen mixture).
The winding permitted a reduction of the filament thermal losses, while
the surrounding gas lowered its evaporation rate. Both combined, this gave
a lamp efficacy of 12 lm/W (first marketed by GE in 1913 in 500, 700 and
1000W sizes) and spelled
the end
of all carbon and other straight filament lamps.
From
this point on, the development of incandescent sources slowed down. In
1933, the first coiled-coil tungsten filament lamp was made available for
general lighting, although it was already in use since 1913 for projection
purposes. The following years saw the introduction of krypton and
xenon-filled lamps having higher filament temperatures owing to reduced
evaporation rates. The impact of these later lamps was limited because the
use of heavier gases did no lead to an efficacy increase higher than ten
percents.
The
last major advance in this domain happened at the end of the 1950’s with
the making by Zuber and Mosby (GE) of the first viable tungsten lamp
having a filling of halogens. The presence of this class of elements
allows a chemical cycle to return evaporated tungsten atoms back to its
source. This permitted the use of ultra-compact packages with 100% lumen
maintenance throughout lamp life (no bulb blackening). Also, its efficacy
was raised to 20 lm/W
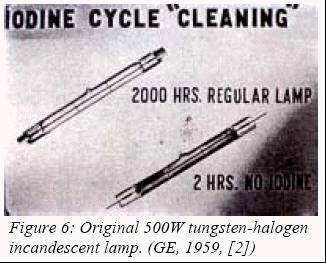 an
later to 26 lm/W, thus making the most efficient incandescent lamp yet.
These sources were first marketed in 1962 and triggered an explosive
development of compact lamps for general, studio, automotive, flood
lighting and movie projection. In the 1980’s the first low voltage capsule
lamps integrated or not in compact reflectors were put on the market,
while infrared-reflecting coatings were tried at the beginning of the
1990’s in an attempt to further decrease the thermal losses of the
filaments.
an
later to 26 lm/W, thus making the most efficient incandescent lamp yet.
These sources were first marketed in 1962 and triggered an explosive
development of compact lamps for general, studio, automotive, flood
lighting and movie projection. In the 1980’s the first low voltage capsule
lamps integrated or not in compact reflectors were put on the market,
while infrared-reflecting coatings were tried at the beginning of the
1990’s in an attempt to further decrease the thermal losses of the
filaments.
Their
pathetic efficacies make incandescent lamps more suitable for heating
purpose than lighting. However, low production costs and simplicity of use
(no current-limiting ballast required) ensures them several decades of
strong use at home and for commercial lighting. If the future see the
development of stable up-converting phosphors transforming infrared into
visible light, or that of proper tungsten optical band-gap crystals, then
incandescent sources will be able to compete with vapor discharge lamps.
The rise
of electric discharge and arc lighting
The
only practical light sources worked out until 1860 where of incandescent
nature. Even the brilliant carbon arc emits its light mainly from the
white-hot anode; the contribution from the arc being relatively
negligible. This year, on September 3
rd , the Hungerford
suspension bridge
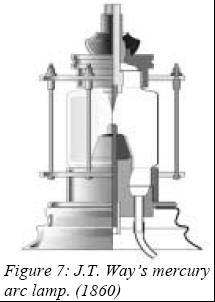 in
London was lighted with the first mercury arc lamps ever made. This
invention from J.T. Way was a carbon arc enclosed in an atmosphere of air
and mercury vapor. This was the first time the arc itself was the source
of light. Mercury in light sources poses today an environmental threat and
work are carried out to suppress it. By then it made a lot of sense to use
it, as this is the only metal with an appreciable vapor pressure at room
temperature and can emit a large proportion of visible light when
energized in electrical discharges. This known fact led to the invention
of the low-pressure mercury lamp by Peter Cooper-Hewitt (USA) in 1901,
followed by a quartz atmospheric-pressure version by R. Küch and T.
Retschinsky (Germany) in 1906 (marketed in 1908 by Westinghouse).
in
London was lighted with the first mercury arc lamps ever made. This
invention from J.T. Way was a carbon arc enclosed in an atmosphere of air
and mercury vapor. This was the first time the arc itself was the source
of light. Mercury in light sources poses today an environmental threat and
work are carried out to suppress it. By then it made a lot of sense to use
it, as this is the only metal with an appreciable vapor pressure at room
temperature and can emit a large proportion of visible light when
energized in electrical discharges. This known fact led to the invention
of the low-pressure mercury lamp by Peter Cooper-Hewitt (USA) in 1901,
followed by a quartz atmospheric-pressure version by R. Küch and T.
Retschinsky (Germany) in 1906 (marketed in 1908 by Westinghouse).
These
lamps performed stunningly well by 1900 standards, they had efficiencies
many times that of carbon filament lamps. The reason for this resides in
the light emission mechanisms that are different in these two kinds of
lamps. Incandescence arises from high thermal energy (i.e. lattice
vibrations in the filament material) that allows the emission of visible
light. Consequently, a large portion of the emitted radiation is in the
infrared (95% of input energy in standard filament lamps). As opposed to
this, an electric discharges and arcs emit their light upon excitation and
relaxation of gas or vapor atoms and molecules from electron impacts. Thus
more input energy can be radiated into useful visible light, leading to
much higher efficiencies (e.g. 35% visible light for low-pressure sodium
vapor). However, the difference between the two kinds of light sources
lies also in their emission spectra. If incandescent lamps give excellent
light color renditions, electric discharge lamps at this time did not.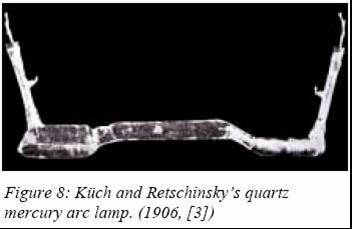
It was
recognized that Cooper-Hewitt and Küch- Retschinsky lamps emitted a bluish
light deficient in red, thus having poor color rendering properties. This
limited their use to streets, warehouses and industries. This particular
problem was addressed with series connected filament lamps that provided
the additional red light and stabilized the electrical discharge.
The
extensive use of both types of mercury lamps started when proper
electrodes were developed. Until the 1930’s, the original lamps had
electrodes made of mercury pools, which waste a lot of electrical energy
for the supply of electrons to the discharge.
High-pressure mercury lamps – the forerunners
The
lamp from Küch and Retschinsky had a limited success due to many unsolved
problems, like proper electrodes, no tight quartz-to-metal seals and
strong UV emissions leading to skin injuries. In the beginning of the
1930’s, many lighting companies worked to address these problems and aimed
at presenting an atmospheric-pressure mercury lamp on the market.
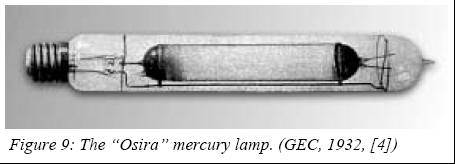 In
1932, General Electric Company of England (GEC) was the first to present
such a lamp under the trade name “Osira”.
In
1932, General Electric Company of England (GEC) was the first to present
such a lamp under the trade name “Osira”.
Because
no satisfactory sealing technique between quartz and tungsten was found,
this lamp used a discharge tube made of aluminosilicate hard glass. The
relatively low softening temperature of this material limited the power
loading of the electric arc to 10-100 W/cm and restricted its use to the
vertical position. This
 later
problem was eventually solved by the use of an electromagnet that kept the
arc straight when the lamp was horizontally operated.
later
problem was eventually solved by the use of an electromagnet that kept the
arc straight when the lamp was horizontally operated.
The
efficacy of such lamp was 30 to 40 lm/W, with a lifetime of a couple of
thousands hours. The low power loading of the arc and the subsequent
electrode power losses did not allow the making of efficient low-power
mercury lamps. Only the 400 and 250W sizes were made available in this
configuration. Also worth of interest, these original lamps did not
integrate any starting aid, like an auxiliary probe. Thus GEC fitted each
luminary with a small Tesla coil in order to ignite the lamp. This was
certainly the first time that an igniter was used.
By the
end of the 1930’s, Willem Elenbaas (Philips, the Netherlands)
theoretically predicted a rise of mercury lamp efficacy with the increase
of the arc power loading. This was effectively verified after the
invention of quartz-to-tungsten graded seals in 1935 (20 atmosphere) lamp,
the HP300 (75W). This was followed by a breakthrough source: the
water-cooled SP500W working at 80 atmospheres (Philips). Not only these
lamps had a better efficacy (40 and 60 lm/W respectively), they also
showed improved color rendering properties owing to the higher operating
pressure.
The
SP500W lamp was primarily designed and used for film projection and
floodlighting applications, while the HP300 remained favored for street
and industrial lighting due to its still insufficient emission of red
light. This problem of color rendering pushed the research toward color
improved lamps that used an integrated incandescent filament (acting also
as a ballast - 1941) and/or a phosphor coating on the inner surface of the
outer bulb to transform useless ultra violets into red light, thus filling
the gap in the mercury spectrum.

In
1934, cadmium sulfide was found to be a suitable fluorescent material,
although it provided only a mild color correction. The introduction of the
color-corrected mercury lamp was made possible with the elaboration of
manganese activated magnesium germanate and fluorogermanate in 1950, which
improved greatly the color rendering index and had a beneficial effect of
the lamp efficacy. Three years later, tin-activated orthophosphate was
introduced, and in an attempt to have proportionally more red emission,
“deluxe” lamps with a rosy glaze on the outer bulb were marketed for a
short while by a number of manufacturers (1956).
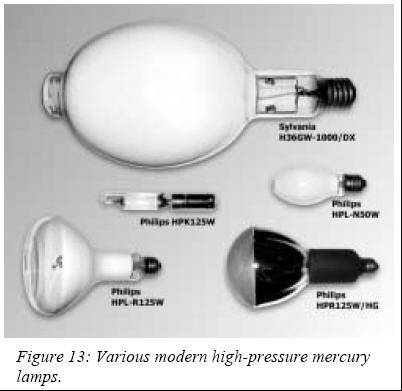
Then in
1967, the hugely successful europium-activated vanadate and
phospho-vanadate phosphors inherited from color TV technology were
introduced and are still in use today. These modern color improved mercury
lamps have a color rendering index (CRI) of 65 against 15 for clear lamps
and a luminous efficacy of 60 lm/W.
The
present design results from a large number of improvements in the lamp
structure that occurred in the 1950’s and 1960’s.
Among
them are new kinds of quartz-to-metal seals using 20 micron-thick
molybdenum foils pressed in quartz. Also, the changeover from thorium to
alkali oxide electrodes (Osram, Germany) permitted a better lumen
maintenance throughout lamp life.
The
last major innovation concerning these lamps occurred in 1998 with the
invention of UHP (Ultra High Performance/Pressure) lamps by Hanns Fischer
(Philips) for LCD projection purposes. These new sources operate with an
internal pressure of about 200 atmospheres, thus leading to a strong
continuum in the emission spectrum and a high arc power loading. These
make this kind of lamp efficient (60 lm/W) and optically small (0.7 mm arc
gap), thus allowing for an excellent optical control.
Standard high-pressure mercury lamps (not UHP) are today on the brink of
extinction because of the environmental threat posed by mercury, and their
relatively poor performances compared to metal halide and high-pressure
sodium sources.
Metal
halide lamps – the legacy of mercury sources
It was
recognized since the earliest days of mercury lamps that the lack of red
light in their emission spectrum impeded heavily on their widespread use.
In 1906, Guercke already suggested to add some red emitting metals to the
lamp of Küch and Retschinsky in order to improve its color properties. M.
Wolke followed this procedure in 1912 and used cadmium and zinc. This
turned out to be unsuccessful due to a low lamp cold-spot temperature
(600ºC), which led to an insufficient zinc and cadmium vapor pressures.
Also, these metals readily attacked the quartz envelope, thus rendering
the lamp useless after a couple of tens of hours of operation.
 The
development of suitable fluorescent materials and ballasting filaments
dampened the need for color improved mercury arcs. However, studies were
still going on possible additives for the mercury lamp in order to
increase its luminous efficacy, regardless of color properties. In 1941,
Schnetzler made a mercury-thallium lamp having an efficiency of 70 lm/W,
almost twice as high as its mercury counterpart. The desired thallium
vapor pressure was reached by operating the arc tube at thrice its normal
power
The
development of suitable fluorescent materials and ballasting filaments
dampened the need for color improved mercury arcs. However, studies were
still going on possible additives for the mercury lamp in order to
increase its luminous efficacy, regardless of color properties. In 1941,
Schnetzler made a mercury-thallium lamp having an efficiency of 70 lm/W,
almost twice as high as its mercury counterpart. The desired thallium
vapor pressure was reached by operating the arc tube at thrice its normal
power
loading, with the consequence we can imagine on the life expectancy. In
the next decade, studies turned toward metal-halogen compounds that have
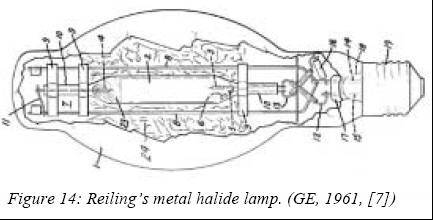
higher vapor pressures than metals at a given temperature. Gilbert Reiling
(GE) patented the first metal halide lamp in 1961, which was intended to
replace high-pressure mercury lamps in their sockets. It had a filling
primarily of mercury, thallium and sodium iodide that showed a sizeable
increase of lamp efficacy (up to 100 lm/W) and color properties, and made
it more suitable for commercial, street and industrial lighting.
Eventually GE marketed this lamp in 1964 with additives of sodium and
scandium iodides instead.
Most
major manufacturers followed shortly thereafter, with varied compositions
in order to meet different lighting needs and to circumvent competitors’
patents. Today the most popular additives are sodium-scandium iodides,
lithium soitum-thallium-indium halides and several mixtures of rare-earth
halides.
The
sixties and seventies witnessed a furious development of metal-halide
lamps in different geometries from tubular to reflector, and in power
range between 175W and 5000W in order to meet the soaring demands in the
many applications it found. One of the last strongholds this kind of
source did not invade was at home. At the end of the 1970’s GE, Sylvania
(USA) and Philips designed prototypes of self-ballasted metal-halide lamps
intended to replace standard filament lamps for domestic applications.
This was ultimately proven unsuccessful due to some lethal drawbacks such
as the lack of hot re-strike capabilities and the prohibitive cost of the
lamps.
Two
major breakthroughs followed at the beginning of the 1980’s. In 1981,
Thorn Lighting (England) presented the first metal halide lamp with a
sintered alumina ceramic discharge tube, which resulted from ten years of
research and development. Unfortunately, this revolutionary source did not
reach the market due to a lamp voltage/current characteristic that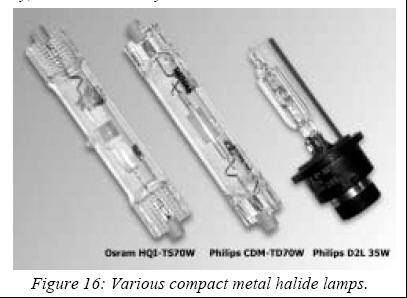 did
not match any available ballast. Around the same year, and with more
success, Osram introduced its compact double-ended HQI-TS lamps that found
an application in shop-window and commercial lighting.
did
not match any available ballast. Around the same year, and with more
success, Osram introduced its compact double-ended HQI-TS lamps that found
an application in shop-window and commercial lighting.
In
1991, Osram, Philips, Valeo and many other car equipment manufacturers
engaged themselves in the ‘vedilis’ project, which led to the
xenonmetal halide lamps (D1 and D2) for automotive headlights. Philips
then revived metal halide lamps with ceramic discharge tubes in 1995, when
it launched its range of CDM lamps. Osram and GE soon followed. These
lamps present today an alternative to high-pressure sodium sources for
downtown street lighting. The use of this particular design allows for a
better lamp-to-lamp color matching, higher efficacies and better color
rendering. Even more so, the bluish light of metal halide performs better
than the orange hue of high-pressure sodium when scotopic vision prevails
in low illumination levels at night.
Low-pressure mercury
fluorescent lamps – toward domestic applications
The
origin of fluorescent tubes goes back to the invention in 1901 of the
low-pressure mercury lamp by Cooper-Hewitt. For the same reasons as its
high-pressure counterpart, its use was restricted to places where color
rendering was not an issue. Right from the start, Cooper-Hewitt worked to
improve his lamp by applying some fluorescent dyes (primarily Rhodamine B)
on the bulb surface and later on luminary reflectors in order to
compensate for the lack of red emission. The idea of using fluorescence to
convert invisible light into useful radiation was not new, and already in 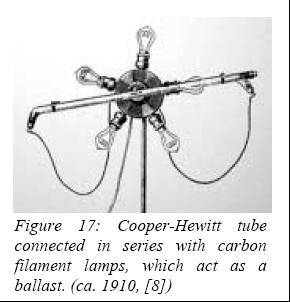 1859
E. Becquerel tried to use Geissler tubes filled with fluorescent materials
in order to get a practical light source. His trials were not successful
as the efficacy was too low. Later in 1896, one year after the discovery
of X-rays by W. Röntgen, T. Edison made a X-ray lamp internally coated
with calcium tungstate which radiated a bluish white light. This source
was three times as efficient as carbon filament lamps, and had X-rays not
caused severe injuries, this lamp would have certainly been the first
commercial fluorescent source.
1859
E. Becquerel tried to use Geissler tubes filled with fluorescent materials
in order to get a practical light source. His trials were not successful
as the efficacy was too low. Later in 1896, one year after the discovery
of X-rays by W. Röntgen, T. Edison made a X-ray lamp internally coated
with calcium tungstate which radiated a bluish white light. This source
was three times as efficient as carbon filament lamps, and had X-rays not
caused severe injuries, this lamp would have certainly been the first
commercial fluorescent source.
Back to
the twentieth century, it was discovered in 1920 that an electrical
discharge in a proper mixture of argon and mercury at low pressure could
radiate efficiently (60% of power input) ultraviolet light at 253.7nm and
184.9 nm. Six years later, Meyer, Spanner and Germer from Osram (Germany)
published a landmark report where they described a low-pressure mercury
vapor lamp provided with externally-heated oxide-coated electrodes, and an
internally phosphor-coated bulb to convert UV radiation into visible
light. This document set what would become the first successful
fluorescent tubes.
However, its marketing had to wait for the development of efficient
electron-emitting electrodes by M. Pirani and A. Rüttenauer (Osram) in
1932, and the elaboration of the calcium tungstate - zinc silicate
phosphor. Then in September of 1935, the first tubular fluorescent lamp
was demonstrated before the Illuminating Engineering Society in
Cincinnati, North America. This was presumably from General Electric, who
had taken over the patent of André Claude on a similar fluorescent tube in
1932. Osram followed in 1936, and displayed its ‘L’ lamp at the World
Exhibition held in Paris. Between 1936 and 1938, most major lamp
manufacturers made fluorescent tubes available both in Europe and in the
US for general lighting applications. These lamps had a tube diameter of
38mm, an efficiency of about 30 lm/W and a moderate color-rendering index,
yet good enough for its use at home.

In
1942, A.H. McKeag from GEC (England) made a giant leap with the discovery
of calcium and strontium-activated halophosphates. Lamps using this
phosphor formulation
were introduced in 1946 and had twice the efficacy of former tubes, while
the color rendering was much improved.
formulation
were introduced in 1946 and had twice the efficacy of former tubes, while
the color rendering was much improved.
Philips
made the next step with the introduction in 1973 of the three-band
phosphors. This boosted the efficacy up to 90 lm/W with excellent color
rendition (IRC 80-90). This new formulation also allowed the increase of
the lamp wall power loading and led to the reduction of the tube diameter
from 38mm (T12) to 26mm (T8), and then to 16mm (T5) at the beginning of
the 1980s.
A
decade later, Osram shrunk things further and put a 7mmdiameter (T2)
fluorescent tube on the market (Lumilux-FM).
The
reduction of lamp size permitted the design of compact fluorescent lamps
with integrated ballast. The first of this kind was presented by Philips
at a world technical conference held in Eindhoven in 1976. In 1980, this
company introduced successfully its SL*18, followed by an electronic
version in 1982. Competitors were quick to catch up and by the end of the
1980’s compact fluorescent lamps were widely available at a reduced cost
and package size. The success of these lamps was partly due to the energy
crisis that raised the cost of electric
 consumption,
thus calling for more efficient and cost-effective light sources.
consumption,
thus calling for more efficient and cost-effective light sources.
At last
and not least, from the eighties until the mid-nineties several lamp
makers introduced electrodeless versions of fluorescent lamps. In these
sources the discharge originates from an electromagnetic field generated
by an induction coil antenna. The suppression of the electrodes increases
the lamp lifetime up to sixty to a hundred thousands of hours.
Fluorescent lamps were the first and only discharge lamps to reach the
level of domestic lighting. Today, they provide a wide range of color
temperature with excellent color rendition and high efficacies.
This
explains why they account for seventy percent of all lamps used in
commercial illuminations. Development still continues today, and
priorities are set to size and efficiency. To this respect, the use of
surface-mounted electronic components permitted the making of smaller CFL
lamps to fit in low-power luminaries, therefore claiming more ground to
its incandescent counterpart.
Low-pressure sodium lamps – reaching summits in efficacy
Extensive experiments with electrical discharges in alkali vapors could
have started only in 1920 when A.H. Compton formulated a borate glass
resistant to sodium. Alkalis, being strong reducers, require special
glasses as normal materials like soda-lime silicates are readily attacked
and lead to the formation of a brown light-absorbing film. Two years
later, in 1922, M. Pirani and E. Lax from Osram experimented sodium
discharges for lighting applications. The following year, Compton and C.C.
van Voorhis in the USA attained an efficacy of 340 lm/W with a lamp
externally heated by an oven. Naturally, the calculation of the efficacy
did not take into account the energy provided to keep the lamp at its
optimum working temperature of 260°C.
Then,
in 1931, both Philips and Osram made the first viable
 low-pressure
sodium lamps, and the following year a stretch of road between Beek and
Geleen, in the Netherlands was lighted with Philips lamps. These sources
were DC-operated via an externally heated cathode and had an efficacy of
about 50 lm/W. A Dewar flask surrounded the discharge tube in order to
limit the thermal losses. In 1933 followed the AC-driven positive column
type of lamp, which had a higher efficacy partly due to a more favorable
current density in the discharge.
low-pressure
sodium lamps, and the following year a stretch of road between Beek and
Geleen, in the Netherlands was lighted with Philips lamps. These sources
were DC-operated via an externally heated cathode and had an efficacy of
about 50 lm/W. A Dewar flask surrounded the discharge tube in order to
limit the thermal losses. In 1933 followed the AC-driven positive column
type of lamp, which had a higher efficacy partly due to a more favorable
current density in the discharge.
From
1933 until 1958, lamps were composed of a separate discharge tube and a
double-walled vacuum flask. In 1958, Philips marketed an integral lamp,
which included the discharge tube within an evacuated bulb thus preventing
the former from getting dirty, as it was the case in the previous design.
Subsequent work was done on increasing the lamp efficacy by improving its
thermal insulation. A first solution consisted of enclosing the discharge
tube in several infrared-absorbing glass sleeves. Then infrared mirrors
made of gold or bismuth
thin
films were employed. Philips
 made
a leap forward in 1965 with the introduction of the tin oxide
semiconductor mirror, and later the better tin-doped indium oxide film.
These materials exhibit a strong infrared reflectivity while being highly
transparent to sodium light.
made
a leap forward in 1965 with the introduction of the tin oxide
semiconductor mirror, and later the better tin-doped indium oxide film.
These materials exhibit a strong infrared reflectivity while being highly
transparent to sodium light.
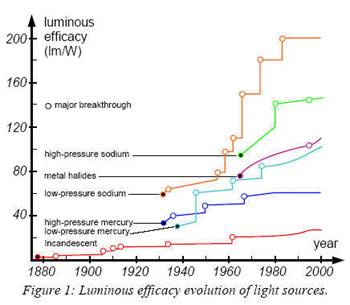
This
led in 1983 to a lamp reaching the symbolic barrier of 200 lm/W (SOX-E, by
Philips), which is the highest efficacy reached yet.
The
reason why low-pressure sodium is so efficient at producing visible light
is that this element, under the right conditions, radiates an
almost-monochromatic yellow light almost coinciding with the peak
sensitivity of the human eye in photopic vision. Also, this yellow light
emission corresponds to transitions from the two lowest (resonant) energy
levels of sodium, thus allowing an efficient transfer of energy from the
electric discharge to the excitation of sodium atoms.
In the
1980s, several low-power lamps were experimented for replacing filament
lamps in security lighting. Technically these sources were successful but
their prohibitive cost and the need for specific ballasts prevented their
widespread use. Interestingly, Philips designed a LPS lamp that had
electrical characteristics closely matching that of existing fluorescent
tubes, so the ballasting equipment was already standard.
Today,
low-pressure sodium lamps remain unchallenged in terms of luminous
efficacy. Its bi-chromatic orange spectrum is the key to its efficiency,
but is also the limitation factor that restrains its use for street and
industrial lighting. In return, its light leads to excellent seeing
contrasts, particularly in foggy weather. Further developments of these
sources concern its high frequency operation and improvement of thermal
insulation, which will certainly bring the efficacy up to 230 lm/W in a
more or less distant future. Also worth of interest is the development of
electrodeless versions that obviate the need for life-limiting parts such
as the electrodes. These sources are however not likely to reach the
market due to difficulties in the making of a proper discharge vessel.
High-pressure sodium lamps – a compromise between color and efficacy
It was
known that increasing the pressure of a sodium discharge would lead to a
lower efficacy, but also to a broader and richer spectrum having better
color-rendering properties. The borate glass originally developed by
Compton and the other materials used in low-pressure sodium lamps are not
suitable for high-pressure operation. As the power loading and the
temperature of the discharge increase, the reactivity of sodium toward the
wall increases and lamps degrades
themselves within minutes of operation. Then, the development of the
high-pressure sodium (HPS) lamp had to wait for the work of Cahoon and
Christensen in 1955-57, and that in 1955 of R.L. Coble on tubes made of
sintered translucent alumina. This material was found to be resistant and
impermeable to alkalis, thus making it suitable for high-pressure sodium
lamps.
degrades
themselves within minutes of operation. Then, the development of the
high-pressure sodium (HPS) lamp had to wait for the work of Cahoon and
Christensen in 1955-57, and that in 1955 of R.L. Coble on tubes made of
sintered translucent alumina. This material was found to be resistant and
impermeable to alkalis, thus making it suitable for high-pressure sodium
lamps.
During
the following years, systematic studies were carried out on high-pressure
alkali discharges. Among them, cesium looked promising due to its
relatively white spectrum. However, the final choice was sodium because of
its good compromise between efficacy
and color rendering. The development of suitable sealing and manufacturing
techniques allowed William Louden and Kurt Schmidt (GE) to make the first
practicable high-pressure sodium lamps in 1964. The next year, GE launched
an industrial full-scale production and a 400W lamp was made available in
1966 under the ‘Lucalox’ brand name. A 250W version followed three years
later. Their efficacies ranged between 90 and 100 lm/W with a life
expectancy of 6000 hours. Refinements in the 1980’s extended the lifetime
to 24,000 hours and the efficacy between 100 and 140 lm/W with a
color-rendering index of 20-25.
efficacy
and color rendering. The development of suitable sealing and manufacturing
techniques allowed William Louden and Kurt Schmidt (GE) to make the first
practicable high-pressure sodium lamps in 1964. The next year, GE launched
an industrial full-scale production and a 400W lamp was made available in
1966 under the ‘Lucalox’ brand name. A 250W version followed three years
later. Their efficacies ranged between 90 and 100 lm/W with a life
expectancy of 6000 hours. Refinements in the 1980’s extended the lifetime
to 24,000 hours and the efficacy between 100 and 140 lm/W with a
color-rendering index of 20-25.
The
design of this lamp was radically different than that of metal halide and
the mercury lamps. It also called for different types of ballasts. While
metal halide and mercury sources were and still are powered in the USA
with step-up leakage transformers, high-pressure sodium lamps required a
choke and an external igniter. Then followed several versions of lamps
with built-in internal switches that use the inductance of the choke to
kick-start the discharge tube.
Most of
these sources have a filling of mercury, xenon and sodium. The role of
xenon is to allow the lamp to start, while mercury sets the electric field
in the lamp discharge (positive column) and does not contribute to the
emission spectrum. Without it, the lamp voltage drop would be too low and
the current too high, thus requiring an inefficient and bulky ballast and
impairing the luminous efficacy. The environmental problems caused by
mercury forces its suppression, and mercury-free HPS lamps were made
available by mid 1990’s. These lamps have a higher xenon pressure and some
starting aid like sintered metal strips on the discharge tube surface
(Philips).
The
1980’s saw also the development of the so-called white HPS lamps by
Thorn, Philips and Iwasaki (Japan), which provide an incandescent-like
color at four times the efficacy of tungsten filament lamps.
These
sources are still popular today even with the advent of ceramic metal
halide lamps. The advantages of white HPS lies in the large portion of red
light in its emission spectrum, leading to a color temperature as low as
2500K. Metal halide lamps cannot reach such war white tone. Also worth of
notice is a lamp developed in the mid-1990s by Osram (DSX-T), which has
its color temperature that can be changed from 2700K (standard tungsten
white) to 2900K (tungsten halogen white) by a flick of a switch.
Bright
perspectives
The
field of lighting had many changes since the revolution in lifestyle and
lightstyle Davys’s discoveries induced! So affected has been and still is
the field of lamp manufacturing. The eighteenth century witnessed the slow
emergence of precursors that led to the exponential development of myriads
of sources in the next hundred years. By the dawn of the twentieth
century, thousands of lamp makers were struggling on a boiling market, and
to say the truth, it was not far from easy to jump in this business since
techniques and physics involved at this time were not as developed as
today. A century later, only three major (general) manufacturers have
survived: Philips, Osram and GE, who count more than 3500 references in
their product catalogs. A couple of hundred of medium-sized, minor or
specialized manufacturers surround them. They are now facing new
challenges that will change our lifestyle and lightstyle through the 21st
century: the development and extensive use of white LEDs, and the abandon
of harmful materials in all vapor discharge lamps while still pushing
upward their luminous efficacies and color rendering properties.
References
More
technical and historical details are available at:
http://www.geocities.com/mfgendre
[1]
F.J.M. Bothe, AEG-Telefunken Ontladingen/Schakels, 57 p., June
1979.
[2]
“Lighting progress in 1959”, Illuminating Engineering, pp.140,
March 1960.
[3] R.
Küch and T. Retschinsky, “Photometrische und spektralphotometrische
messungen am quecksilberbogen bei hohem dampfdruck”, Annalen der Physik,
vol. 20, pp. 563-583, June 1906.
[4]
C.C. Paterson, “Luminous discharge tube lighting”, The journal of good
lighting, pp.308-318, December 1932.
[5]
P.J. Oranje, Gasontladingslampen, Uitgave Meulenhoff & Co.,
Amsterdam, 288p., 1942.
[6]
J.L. Ouweltjes, W. Elenbaas and K.R. Labberté, “A new high-pressure
mercury lamp with fluorescent bulb”, Philips Technical Review, no.
5, pp. 109-144, November 1951.
[7]
G.H. Reiling, “Metallic halide discharge lamps”, US patent #3,234,421,
January 23rd, 1961.
[8] G.W. Stoer,
History of lights and lighting, Philips Lighting B.V., the Netherlands,
46 p., 1988.
[9] “Fifty years of
low-pressure sodium lighting”, Philips Lighting News, no. 8, 1982.
[10] K. Schmidt, “Metal
vapor lamps”, US patent #2,971,110, February 7th,
1961.
[11] W.C. Louden and
W.C. Matz, “High-intensity sodium lamp design data for various sizes”,
Illuminating
Engineering , pp.
560-561, September 1966.